Chlamydophila felis: A Unique Bacteria Causing Diseases to Felines
Maigan Espinili Maruquin
Structure and Replication
The chlamydiae is unique obligate intracellular bacteria. The Chlamydophila felis is a Gram- negative and rod- shaped coccoid bacterium however the cell wall lacks peptidoglycan (Gruffydd-Jones, Addie et al. 2009). It has two morphologically distinct structures. The (1) EB or elementary body is metabolically inert infectious, round and small (~0.3 μm), and is responsible for its survival in extracellular environment with its ‘spore-like’ form with a rigid cell wall. It holds the central and dense nucleoid. Whereas, the other form (2) RB or the replicative but noninfectious reticulate body which is larger (~1 μm) than the EB. It has cross-linked membrane proteins which makes it structurally flexible and osmotically fragile. It contains RNA and diffuse and fibrillary DNA, allowing intracellular replication, nutrient uptake and transportation, protein synthesis and other metabolic activities (Bedson and Bland 1932, Moulder 1991, Nunes and Gomes 2014).
The chlamydiae is unique for its biphasic developmental cycle of 30–72 hours (Nunes and Gomes 2014). This bacteria, during its intracellular life, stays in a parasitophorous vacuole, or inclusion to acquire the nutrition it needs. (Hybiske and Stephens 2007). First, the EB attaches and enters the host cell which leads to formation of vacuole. Inside the inclusions, the EB differentiates from the RB. The RB then replicates via binary fission (Borges, V. et. al., 2013) (Nunes and Gomes 2014). The inclusions then expand while RB undergoes transition or conversion back to EB. Finally, the bacteria is released through host cell lysis or via extrusion (Hybiske and Stephens 2007, Nunes and Gomes 2014).
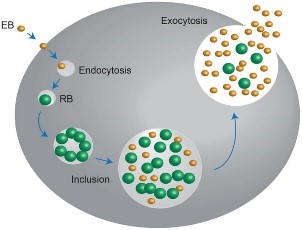
Fig. 01. The unique biphasic developmental cycle of Chlamydiae
(Source: https://www.researchgate.net/figure/Chlamydia-undergo-a-unique-biphasic developmental-cycle-The-infectious-form-of_fig3_268229035 )
Chlamydophila felis Epidemiology
The Chlamydiaceae is reported to have cause animal infection often indirectly and associated with other pathogens (Schautteet and Vanrompay 2011, Nunes and Gomes 2014). The Chlamydophila felis grows in the cytoplasm of epithelial cells and produces inclusion bodies (Halánová, Sulinová et al. 2011). The C. felis requires close contact between cats to transmit while ocular secretions are considered the most important body fluid for the infection. The disease caused by the C. felis is common in multi- cat environments (Wills JM et al., 1987)(Gruffydd-Jones, Addie et al. 2009) while it is also frequently associated with conjunctivitis (WILLS, HOWARD et al. 1988, Gruffydd-Jones, Addie et al. 2009). Despite the low zoonotic potential, possible exposure to the C. felis is through handling of infected cats, by contact with their aerosol and also via fomites (Baker 1942, Halánová, Sulinová et al. 2011).
Reports in culture and PCR (Sykes, Anderson et al. 1999, Sykes 2005) showed that C. felis most likely to infect cats less than a year of age and less likely for cats age 5 years above. There is no strong breed or sex preference and prevalence of asymptomatic cases are low (Sykes 2005).
Clinical Signs/ Pathogenesis
The C. felis is known to cause conjunctivitis associated with severe swelling of the lid, mild rhinitis, ocular and nasal discharges, fever, and lameness (Masubuchi, K, et al. 2002)(TerWee, Sabara et al. 1998, Rodolakis and Yousef Mohamad 2010). In kittens, chlamydiosis most commonly cause pneumonia and conjunctivitis (TerWee, Sabara et al. 1998, Yan, Fukushi et al. 2000)( Sykes, J. E., 2001)(Halánová, Sulinová et al. 2011) and can cause disease to adults, too (Sykes 2005, Halánová, Sulinová et al. 2011).
While C. felis affects conjunctival epithelial cells, natural transmission occurs by close contact with other infected felines, aerosols, and fomites with approximately 3 to 5 days of incubation period (Sykes 2005, Gruffydd-Jones, Addie et al. 2009). Generally, conjunctival shedding ceases at around 60 days after infection, however some cats may carry persistent infection (O’Dair HA , et al, 1994; Wills JM., 1986;) (Sykes 2005, Gruffydd-Jones, Addie et al. 2009). Due to the reported chlamydial conjunctivitis in the rectal and vaginal excretion from cats, intestinal and reproductive tracts were considered sites for the persistent infections (Wills JM., 1986)(Sykes 2005). On the other hand, findings of the C. felis were also in lung, spleen, liver, kidney and peritoneum of cats (Dickie CW, Sniff ES, 1980; Hoover EA, 1980) (Baker 1944, Masubuchi, Nosaka et al. 2002, Sykes 2005).
Other microorganisms may coinfect C. felis. Felines infected by C. felis show clinical signs including: sneezing, transient fever, inappetence, weight lost, nasal discharge, vaginal discharge, lameness and lethargy (Halánová, Sulinová et al. 2011). Unilateral ocular disease may appear during the first day or two which progresses to bilateral. Discharge in the ocular is watery which becomes mucoid or mucopurulent while chemosis can be observed in the conjunctiva. However, although the cats show symptoms after infection, they mostly still continue to eat (Gruffydd-Jones, Addie et al. 2009).
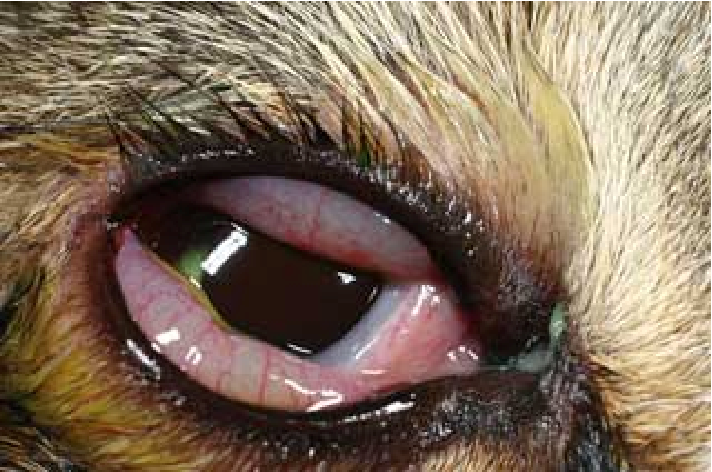
Fig. 2. A young cat from a multi- cat household showed chlamydial conjunctivitis (Heinrich, C., 2017).
(Source: https://www.semanticscholar.org/paper/Bacterial-conjunctivitis-Chlamydophila-felis-Heinrich/12a1d5eee2fbc8121665d94441ff698531c32868 )
Diagnosis
Although the use of indirect immunofluorescence can be used to detect the serum antibody titer, this method should be used after a diagnosis of considerable rise in antibody titer (Sykes 2005, Gruffydd-Jones, Addie et al. 2009). On the other hand, cell culture is considered to be the golden standard in diagnosing chlamydial infections (Pointon AM, et al., 1991) (Wills JM, et. al., 1988) (Sykes 2005). The cell culture technique uses fluorescent antibodies in detecting inclusions. However, cell culture isolation is a demanding, time-consuming and expensive technique while sensitivity of the culture may vary depending on the equipment being used and the technical expertise (Sykes 2005). Further, Giemsa staining can be used for inclusions but this causes confusion with other basophilic inclusions (Gruffydd-Jones, Addie et al. 2009)
Also, conjunctival smears can be Giemsa stained to look for inclusions, but chlamydial bodies are easily confused with other basophilic inclusions (Streeten BW, Streeten EA, 1985) (Gruffydd-Jones, Addie et al. 2009) and inclusions are often seen only on early infection, and at times, they are not visible (Wills JM, 1986)(Sykes 2005).
For a quicker, less expensive and more sensitive diagnosis than cell culture and ELISA, the PCR assay technique can be used, however, proper guidelines on handling should be observed to avoid contamination on the samples(Sykes 2005). PCR techniques is extremely sensitive and ocular swabs are generally used, however, due to its intracellular characteristic, conjunctival swabs should have enough number of cells (Gruffydd-Jones, Addie et al. 2009).
Vaccine and Disease Managements
The chlamydophila infection in felines can be treated with antibiotics (Sykes 2005, Gruffydd-Jones, Addie et al. 2009) like tetracyclines and doxycycline (Dean, Harley et al. 2005), erythromycin, rifampin, fluoroquinolones, and the azalide azithromycin (Stamm WE, 1998) (Sykes 2005).
Periods of longer than 3 weeks sometimes work to eliminate natural infections (Sykes 2005). On the other hand, cattery cats usually take 6 to 8 weeks of treatment (Greene CE, 1998) (Gruffydd-Jones, Jones et al. 1995, Sykes 2005). It is recommended still to continue the treatment for two weeks even after clinical signs were resolved (Sykes 2005).
On the other hand, chronic infections may be hard to eliminate although recurring infections usually involve in large numbers of cats and those with poor compliance. With this, full course of antimicrobials shall be given and proper hygiene and quarantine shall be imposed (Sykes 2005).
With generally weak or short-lived immunity, age-related resistance suggests that some form of protective immunity must eventually develop and both humoral and cell-mediated immunity are needed to solve the infection (Sykes 2005).
Multivalent vaccines with inactivated and modified-live virus vaccines are available on whole chlamydial organisms. For the felines at risk to exposure, vaccination is recommended (Gruffydd-Jones, Addie et al. 2009). In the United States, modified live and inactivated cell culture vaccines have already been used either alone or in combination with feline panleukopenia, FCV and FHV-1 components. Although the vaccine doesn’t prevent infection and clinical signs, vaccines may still be of some benefits to control the infection (Sykes 2005).
References:
- Greene CE: Chlamydial infections, in Greene CE (ed): Infectious Diseases of the Dog and Cat (ed 2). Philadelphia, WB Saunders, 1998, pp 172-174
- Stamm WE: Chlamydial infections, in Fauci AS, Braunwald E, Isselbacher KJ, et al (eds): Harrison’s Principles of Internal Medicine. New York, McGraw-Hill, 1998, pp 1055-1064
- Streeten BW, Streeten EA. ‘Blue-body’ epithelial cell inclusions in conjunctivitis. Ophthalmology 1985; 92: 575–79
- Wills JM, Millards WG, Howard PE: Evaluation of a monoclonal antibody based ELISA for detection of feline Chlamydia psittaci. Vet Rec 119:418-420, 1988
- Pointon AM, Nicholls JM, Neville S, et al: Chlamydia infection among breeding catteries in South Australia. Aust Vet Practit 21:58-63, 1991
- Hoover EA: Feline pneumonitis, in Kirk RW (ed): Current Veterinary Therapy VII. Small Animal Practice. Philadelphia, WB Saunders, 1980, pp 1299-1302
- Dickie CW, Sniff ES: Chlamydia infection associated with peritonitis in a cat. J Am Vet Med Assoc 176:1256-1259, 1980
- Wills JM. Chlamydial infection in the cat. PhD Thesis, University of Bristol, 1986
- O’Dair HA, Hopper CD, Gruffydd-Jones TJ, Harbour DA, Waters L. Clinical aspects of Chlamydia psittaci infection in cats infected with feline immunodeficiency virus. Vet Rec 1994; 134: 365–68
- Sykes, J. E., 2001: Feline upper respiratory tract pathogens: Chlamydophila felis. Comp. Contin. Edu. Pract. Vet. 23, 231–235.
- Masubuchi, K., Nosaka, H., Iwamoto, K., Kokubu, T., Yamanaka, M., Shimizu, Y., 2002. Experimental infection of cats with Chlamydophila felis. J. Vet. Med. Sci. 64, 1165–1168.
- Wills JM, Gruffydd-Jones TJ, Richmond SJ, Gaskell RM, Bourne FJ. Effect of vaccination on feline Chlamydia psittaci infection. Infect Immun 1987; 55: 2653–57
- Borges, V., Ferreira, R., Nunes, A., Sousa-Uva, M., Abreu, M., Borrego, M.J., Gomes, J.P., 1083 2013. Effect of long-term laboratory propagation on Chlamydia trachomatis 1084 genome dynamics. Infect. Genet. Evol. 17, 23–32.
- Baker, J. A. (1942). “A VIRUS OBTAINED FROM A PNEUMONIA OF CATS AND ITS POSSIBLE RELATION TO THE CAUSE OF ATYPICAL PNEUMONIA IN MAN.” Science 96(2499): 475-476.
- Baker, J. A. (1944). “A VIRUS CAUSING PNEUMONIA IN CATS AND PRODUCING ELEMENTARY BODIES.” The Journal of experimental medicine 79(2): 159-172.
- Bedson, S. P. and J. O. W. Bland (1932). “A Morphological Study of Psittacosis Virus, with the Description of a Developmental Cycle.” British journal of experimental pathology 13(5): 461-466.
- Dean, R., R. Harley, C. Helps, S. Caney and T. Gruffydd-Jones (2005). “Use of quantitative real-time PCR to monitor the response of Chlamydophila felis infection to doxycycline treatment.” J Clin Microbiol 43(4): 1858-1864.
- Gruffydd-Jones, T., D. Addie, S. Belák, C. Boucraut-Baralon, H. Egberink, T. Frymus, K. Hartmann, M. J. Hosie, A. Lloret, H. Lutz, F. Marsilio, M. G. Pennisi, A. D. Radford, E. Thiry, U. Truyen and M. C. Horzinek (2009). “Chlamydophila Felis Infection: ABCD Guidelines on Prevention and Management.” Journal of Feline Medicine and Surgery 11(7): 605-609.
- Gruffydd-Jones, T. J., B. R. Jones, H. Hodge, M. Rice and M. A. Gething (1995). “Chlamydia infection in cats in New Zealand.” New Zealand Veterinary Journal 43(5): 201-203.
- Halánová, M., Z. Sulinová, L. Čisláková, A. Trbolová, Ľ. Páleník, T. Weissová, M. Halán, Z. Kalinová and M. Holičková (2011). “Chlamydophila felis in Cats – Are the Stray Cats Dangerous Source of Infection?” Zoonoses and Public Health 58(7): 519-522.
- Hybiske, K. and R. S. Stephens (2007). “Mechanisms of host cell exit by the intracellular bacterium Chlamydia.” Proc Natl Acad Sci U S A 104(27): 11430-11435.
- Masubuchi, K., H. Nosaka, K. Iwamoto, T. Kokubu, M. Yamanaka and Y. Shimizu (2002). “Experimental infection of cats with Chlamydophila felis.” J Vet Med Sci 64(12): 1165-1168.
- Moulder, J. W. (1991). “Interaction of chlamydiae and host cells in vitro.” Microbiological reviews 55(1): 143-190.
- Nunes, A. and J. P. Gomes (2014). “Evolution, phylogeny, and molecular epidemiology of Chlamydia.” Infection, Genetics and Evolution 23: 49-64.
- Rodolakis, A. and K. Yousef Mohamad (2010). “Zoonotic potential of Chlamydophila.” Veterinary Microbiology 140(3): 382-391.
- Schautteet, K. and D. Vanrompay (2011). “Chlamydiaceae infections in pig.” Veterinary Research 42(1): 29.
- Sykes, J. E. (2005). “Feline chlamydiosis.” Clin Tech Small Anim Pract 20(2): 129-134.
- Sykes, J. E., G. A. Anderson, V. P. Studdert and G. F. Browning (1999). “Prevalence of feline Chlamydia psittaci and feline herpesvirus 1 in cats with upper respiratory tract disease.” J Vet Intern Med 13(3): 153-162.
- TerWee, J., M. Sabara, K. Kokjohn, J. Sandbulte, P. Frenchick and K. J. Dreier (1998). “Characterization of the systemic disease and ocular signs induced by experimental infection with Chlamydia psittaci in cats.” Veterinary Microbiology 59(4): 259-281.
- WILLS, J. M., P. E. HOWARD, T. J. GRUFFYDD-JONES and C. M. WATHES (1988). “Prevalence of Chlamydia psittaci in different cat populations in Britain.” Journal of Small Animal Practice 29(6): 327-339.
- Yan, C., H. Fukushi, H. Matsudate, K. Ishihara, K. Yasuda, H. Kitagawa, T. Yamaguchi and K. Hirai (2000). “Seroepidemiological Investigation of Feline Chlamydiosis in Cats and Humans in Japan.” Microbiology and Immunology 44(3): 155-160.

