Trinh Mai Nguyen Tang
Feline herpesvirus-1 (FHV-1) is a feline respiratory infection virus also known as feline viral rhinotracheitis (FVR) [1]. The Herpes virus was first isolated by scientists Crandell and Maurer in 1958 in cats with respiratory infections [2]. This virus has a prominent genome with large double stranded DNA, belonging to the family Herpesviridae [3]. This virus is characterized by cat-to-cat transmission with an exposure rate of up to 97% [4].
Herpes virus can be inactivated at 37oC around 3 hours or at 56oC in 5 mins. Meanwhile, the virus can remains infective in the enviroment approximately 5 months and a month at 25oC [5].
Once a cat is infected with the herpes virus, it is incredibly difficult to completely treat it since the virus can enter a dormant state and continue to survive in the cat for the remainder of its life [6]. Cats are not infectious during this latent period, but if they are sick or going through a stressful period, the virus may be reactivate. If this occurs, the cat will once more get the infection and may represent symptoms [7].
Herpes viruses can be latent in the ganglion, attach to sensory nerves and reach nerve cells, persist in the nucleus of infected nerve cells and do not replicate, leading to the process of detecting this virus becomes difficult [7-8].
As reported by Ngoc.N.T and her colleagues, herpes virus can infect cats of any age, however, kittens are more susceptible [9]. Specifically, the prevalence of virus infection in cats younger than 6 months old, 6-12 months old and over 12 months old were 52.17%, 33.33% and 19.05%, respectively [9].
Although previous reports have demonstrate that gender has no effect on the incidence of herpes virus infection [7-8], but Henzel et al. (2002) found that isolates from female cats are substantially taller than isolates from male cats [10].
Clinical Symptoms
Herpes virus enters the cat’s body by contact with infected tears, nose, saliva, or items, and then multiplies rapidly in the epithelium of the nose, nasopharynx, and conjunctival mucosa leading to primary infection [11].
In cats infected with FHV-1, signs of sadness, moodiness, lethargy, sneezing, fever, and discharge from the eyes and nose have been noted (figure 1-A), a process that is frequently extended 2-4 days or longer, depending on the immunological system of the cat [11].
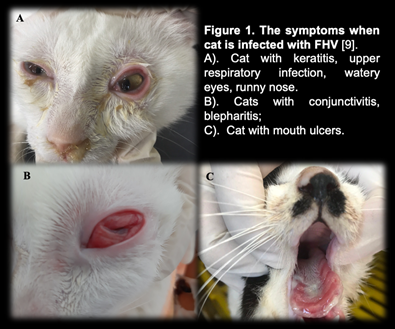
Secondary infection occurred after the fourth day of incubation, with symptoms of infection in the throat, bronchi, and bronchioles, and the nasal and conjunctival epithelium necrosing [12]. Conjunctivitis is a common herpes virus symptom, indicated by congestive and exudative symptoms that develop over many days to purulent discharge (figure 1-B) [13].
Gaskell and Dawson (1988) found lung infection or bronchitis in cats, with kittens dying from pneumonia at a greater incidence than adult cats [11,14].
Some other atypical symptoms such as mouth and skin ulcers, dermatitis or neurological signs are rarely seen [7].
Furthermore, the mean white blood cell (WBC) count of cats infected with FHV-1 (17.77 ± 0.70 x103/μl) was slightly increased compared with that of normal cats (4.6-12.8 x 103/μl) [9], in which neutrophils, eosinophils and monocytes all showed signs of slight increase compared. Secondary infections of the eyes, upper respiratory tract, and necrotic ulcers of the mouth can all cause high white blood cell counts [11]. Table 1 displays the white blood cell count.
Table 1. Hematological results of cats was infected with FHV-1 [9].
| Targets | Unit | Reference | ± SE |
| Red blood cells | ´ 106/μl | 7-10,7 | 10,20 ± 0,64 |
| Hemoglobin content | g/dl | 11,3-15,5 | 13,47 ± 0,54 |
| RBC mass | % | 33-45 | 38,78 ± 1,41 |
| Average volume of red blood cells | fl | 41-49 | 45,16 ± 0,75 |
| Average amount of hemoglobin in red blood cells | pg | 14-17 | 15,57 ± 0,24 |
| Platelet count | ´ 103/μl | 180-680 | 362,50 ± 30,82 |
| WBC count | ´ 103/μl | 4,6-12,8 | 17,77 ± 0,70 |
| Lymphocytes | ´ 103/μl | 1,05-6,00 | 4,50 ± 0,36 |
| Mono leukocytes | ´ 103/μl | 0,05-0,68 | 0,96 ± 0,13 |
| Neutrophils polymorphonuclear leukocytes | ´ 103/μl | 2,32-10,01 | 11,47 ± 0,45 |
| Eosinophils | ´ 103/μl | 0,1-0,6 | 0,78 ± 0,08 |
| Basophils | ´ 103/μl | 0-0,14 | 0,07 ± 0,01 |
Laboratory Diagnosis
In the laboratory, there are many different methods used to determine FHV. Common approaches include PCR, virus isolation in cell culture, and indirect fluorescent antibody staining of tissue samples for viral antibody detection [8,16].
PCR
FHV is one of the most common causes of upper respiratory tract illness in cats. Infected cats would show upper respiratory signs. Co-infection of FHV with other pathogens makes the clinical signs more severe, particularly feline calicivirus, Chlamydophila felis, Bordetella pneumoniaseptica, Mycoplasma species, Staphylococcus spp., or Escherichia coli [6].
When a cat is suspected of having a viral infection, a swab can be used to collect nasal, ocular, oropharyngeal secretions, corneal debris, aqueous humor, corneal samples, blood, or biopsies. By amplifying viral DNA, tPCR can detect genetic material of FHV in specimen. However, if the cat is not in the infectious phase, no virus particles will be shed, rendering the PCR test ineffective [19-20].
ELISA method – detecting IgG antibodies
The enzyme-linked immunosorbent test (ELISA) is used to determine IgG antibodies against FHV or FHV-1 utilizing serum, aqueous humor, and cerebrospinal fluid samples [15]. This approach, however, cannot discriminate between diseased and vaccinated cats. Due to the extended latent period of FHV, the cat’s body will generate antibodies to combat it [8]. These neutralizing antibodies manifest 20-30 days after the first infection. As a result, the presence of antibodies in the serum signals a prior infection but does not always correspond with clinical signs [8].
Immunofluorescent antibody assay
Another approach for detecting FHV is immunofluorescence antibody (IFA) testing on corneal or conjunctival smears or biopsiengs. Through an antigen-antibody response, this assay may identify viral proteins produced in cells. However, this approach is thought to be less sensitive than viral isolation or PCR [16].
Virus isolation
This is a traditional method that can detect viruses through isolation of conjunctival debris, nose, oropharynx, or postmortem lung samples from infected cats [8]. This traditional method can detect the virus by isolating viruses from conjunctival debris, nose, oropharynx, or postoperative lung samples of infected cats [8]. However, this method is not used very often because it is less sensitive than PCR methods [17,18]. Also, this method is only suitable for detecting the virus in the acute phase of the disease [16].
References
[1] Liu, L., Zhou, Y., Ru, Y., Tian, H., Wang, X., Zhang, S., … & Sun, Y. (2022). Duplex real-time qPCR for Feline Herpesvirus-Ⅰ and Feline Parvovirus.
[2] Crandell, R. A., & Maurer, F. D. (1958). Isolation of a feline virus associated with intranuclear inclusion bodies. Proceedings of the Society for Experimental Biology and Medicine, 97(3), 487-490.
[3]Rota, P. A., Maes, R. K., & Ruyechan, W. T. (1986). Physical characterization of the genome of feline herpesvirus-1. Virology, 154(1), 168-179.
[4] Maggs, D. J., Lappin, M. R., Reif, J. S., Collins, J. K., Carman, J., Dawson, D. A., & Bruns, C. (1999). Evaluation of serologic and viral detection methods for diagnosing feline herpesvirus-1 infection in cats with acute respiratory tract or chronic ocular disease. Journal of the American Veterinary Medical Association, 214(4), 502-507.
[5] Pedersen, N. C. (1987). Feline herpesvirus type 1 (feline rhinotracheitis virus). Virus infections of carnivores/edited by Max J. Appel.
[6] Gaskell R, Dawson S& Radford A (2006). Feline respiratory disease. In: Greene CE, ed. Infectious disease of the dog and cat. Missouri: WB Saunders, 145–54.
[7] Gaskell, R., Dawson, S., Radford, A., & Thiry, E. (2007). Feline herpesvirus. Veterinary research, 38(2), 337-354.
[8] Thiry, E., Addie, D., Belák, S., Boucraut-Baralon, C., Egberink, H., Frymus, T., … & Horzinek, M. C. (2009). Feline herpesvirus infection. ABCD guidelines on prevention and management. Journal of Feline Medicine & Surgery, 11(7), 547-555.
[9] Ngọc, N. T., Lê Văn Phan, N. T. H., & Lưu, H. C. Ứng dụng kỹ thuật PCR trong chẩn đoán feline herpesvirus-1 (FHV-1) ở mèo.
[10] Henzel, A., Brum, M. C. S., Lautert, C., Martins, M., Lovato, L. T., & Weiblen, R. (2012). Isolation and identification of feline calicivirus and feline herpesvirus in Southern Brazil. Brazilian Journal of Microbiology, 43, 560-568.
[11] Gaskell RM, Dawson S (1998) In: Infectious Diseases of the Dog and Cat (2nd edn). Greene CE (ed.). W.B. Saunders Company, Philadelphia, pp. 97–106.
[12] Nasisse, M. P. (1990). Feline herpesvirus ocular disease. Veterinary Clinics of North America: Small Animal Practice, 20(3), 667-680.
[13] Andrew, S. E. (2001). Ocular manifestations of feline herpesvirus. Journal of feline medicine and surgery, 3(1), 9-16.
[14] Maggs, D. J. (2005). Update on pathogenesis, diagnosis, and treatment of feline herpesvirus type 1. Clinical techniques in small animal practice, 20(2), 94-101.
[15] Dawson, D. A., Carman, J., Collins, J., Hill, S., & Lappin, M. R. (1998). Enzyme-linked immunosorbent assay for detection of feline herpesvirus 1 IgG in serum, aqueous humor, and cerebrospinal fluid. Journal of Veterinary Diagnostic Investigation, 10(4), 315-319.
[16] Burgesser, K. M., Hotaling, S., Schiebel, A., Ashbaugh, S. E., Roberts, S. M., & Collins, J. K. (1999). Comparison of PCR, virus isolation, and indirect fluorescent antibody staining in the detection of naturally occurring feline herpesvirus infections. Journal of Veterinary Diagnostic Investigation, 11(2), 122-126.
[17] Gaskell, R. M., & Povey, R. C. (1977). Experimental induction of feline viral rhinotracheitis virus re-excretion in FVR-recovered cats. The Veterinary Record, 100(7), 128-133.
[18] Maggs, D. J., Lappin, M. R., Reif, J. S., Collins, J. K., Carman, J., Dawson, D. A., & Bruns, C. (1999). Evaluation of serologic and viral detection methods for diagnosing feline herpesvirus-1 infection in cats with acute respiratory tract or chronic ocular disease. Journal of the American Veterinary Medical Association, 214(4), 502-507.
[19] Marsilio, F., Di Martino, B., Aguzzi, I., & Meridiani, I. (2004). Duplex polymerase chain reaction assay to screen for feline herpesvirus-1 and Chlamydophila spp. in mucosal swabs from cats. Veterinary research communications, 28, 295.
[20] Helps, C., Reeves, N., Egan, K., Howard, P., & Harbour, D. (2003). Detection of Chlamydophila felis and feline herpesvirus by multiplex real-time PCR analysis. Journal of clinical microbiology, 41(6), 2734-2736.

