Spinal Muscular Atrophy in Cats

Bioguard Corporation Spinal muscular atrophy (SMA) an autosomally recessive inherited neurodegenerative disorder seen in Maine Coon cats. The disease is characterized by weakness and atrophy in muscles due to loss of motor neurons that control muscle movement. Affected cats first show signs of disease around 3–4 months of age. Clinical signs include tremors, abnormal posture, […]
Polycystic Kidney Disease in Cats

Bioguard Corporation Polycystic kidney disease (PKD) is a chromosomally dominant genetic disorder; it can occur in humans, cats, dogs, and other animals. In the renal cortex and medulla, there are cysts of various sizes and fluid-filled, so it is commonly known as the bubble kidney. Cysts increase in size and number over time, replacing kidney […]
Feline Hypertrophic Cardiomyopathy
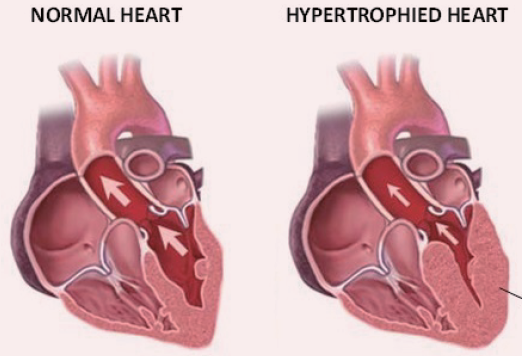
Bioguard Corporation Hypertrophic cardiomyopathy (HCM) is a primary, familial, and hereditary heart condition, and it is the most common heart disease in cats. Its key characteristic is primary concentric left ventricular hypertrophy (thickening of the heart wall), which occurs without pressure overload (such as from aortic stenosis), hormone stimulation (like in hyperthyroidism or acromegaly), myocardial […]
Dog Frostbite: Canine Degenerative Spinal Neuropathy

Bioguard Corporation Degenerative Myelopathy, DM is a progressive chronic degenerative disease of the central nervous system, which is a recessive genetic disease that mainly occurs in the spinal nerve, similar to human amyotrophic lateral sclerosis. It is known as lou gehrig’s disease. The age of onset of dogs is between 9 and 11 years old, […]
GM1 Gangliosidosis in Cats
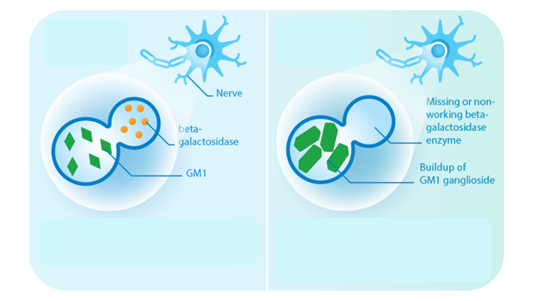
Bioguard Corporation GM1 gangliosidosis is a lysosomal storage disorder caused by deficiency of the enzyme β-galactosidase. Mutations in the GLB1 gene, encoding β-galactosidase, cause the progressive, neurosomatic, lysosomal storage disorder. Cats affected with gangliosidosis have progressive neurologic dysfunction around 3 months of age and premature death around one year old. Pathogenesis Gangliosides, normally hydrolyzed […]
Bird Sexing

Bioguard Corporation Many mammals are sexually dimorphic, which means that their sex can be identified based on their appearance. However, it is not the case for most birds. Many birds are sexually monomorphic although males and females may differ markedly in size, color, and appearance in some birds, such as peacocks (Fig. 1). Knowledge of […]
Severe Fever with Thrombocytopenia Syndrome
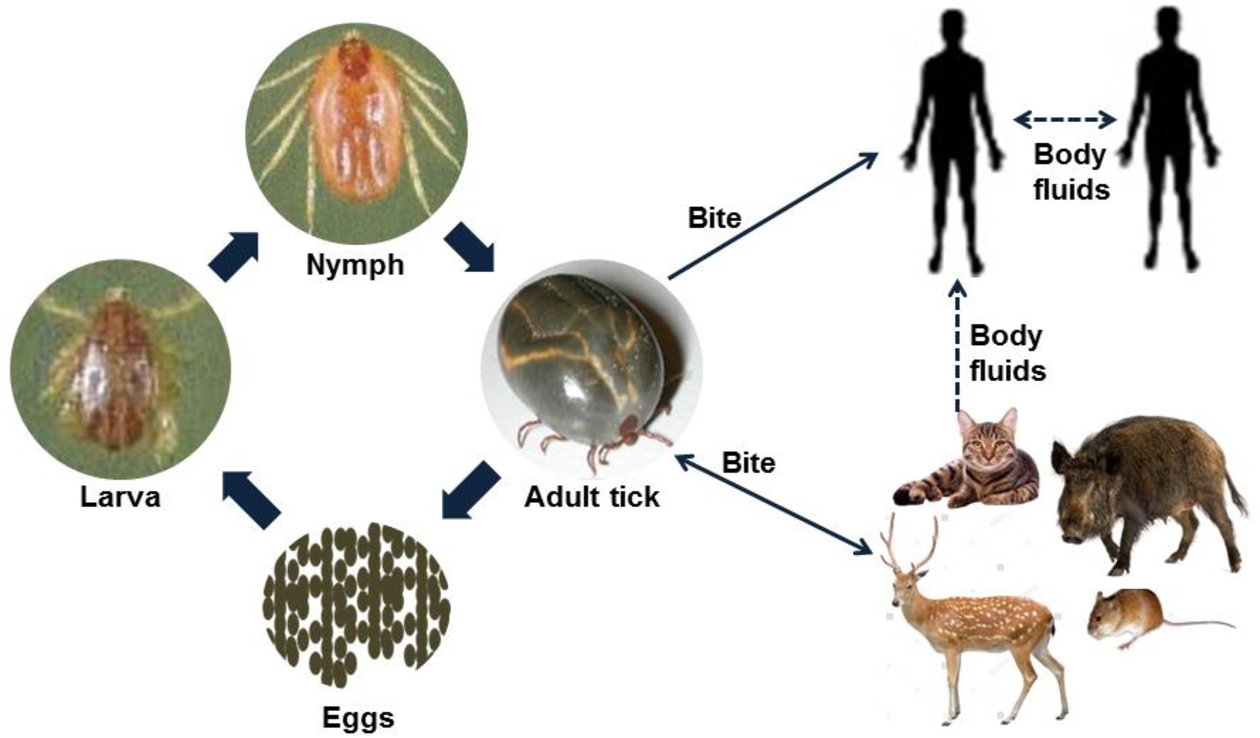
Bioguard Corporation Severe Fever with Thrombocytopenia Syndrome (SFTS) is an emerging infectious disease that was first reported in China in 2011. It is caused by the Severe Fever with Thrombocytopenia Syndrome virus (SFTSV) and is transmitted to humans and animals through the bite of an infected tick. The main clinical symptoms of SFTS include fever, […]
Cryptosporidiosis

Bioguard Corporation Cryptosporidiosis is an illness you get from the parasite Cryptosporidium. It causes watery diarrhea and other gastrointestinal (gut) symptoms. In addition to stomach infection, this parasite can infect the respiratory system causing a cough and/or problems breathing. The family Cryptosporididae belongs to the phylum Apicomplexa characterized by an anterior (or apical) polar complex […]
Ferret Coronavirus

Bioguard Corporation The coronavirus of ferrets was first described in 1993. This ferret enteric coronavirus (FRECV) caused an enteric disease called epizootic catarrhal enteritis (ECE) or green slime disease. Later, a ferret systemic coronavirus (FRSCV)-associated disease, resembling the dry form of feline infectious peritonitis (FIP), was identified. ECE is characterized by high morbidity (nearly 100%) […]
Infectious Myxomatosis of Rabbits
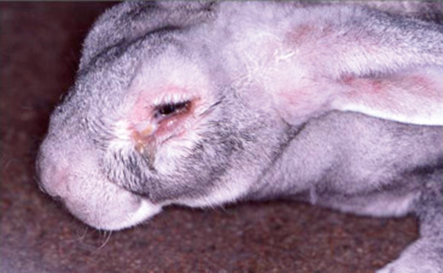
Bioguard Corporation Myxomatosis is primarily a disease of rabbits caused by infection with the myxoma virus. It mainly occurs in domestic and wild rabbits. The virus is harmless to humans. Myxomatosis can result in lumps developing around the ears and face. These lumps are named myxomas and the disease virus was named after this lesion. […]



