Infectious Myxomatosis of Rabbits
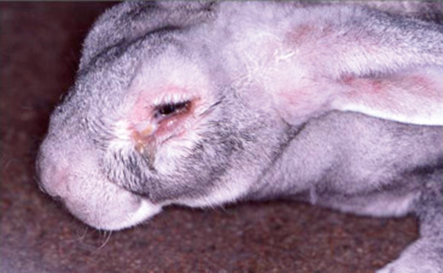
Bioguard Corporation Myxomatosis is primarily a disease of rabbits caused by infection with the myxoma virus. It mainly occurs in domestic and wild rabbits. The virus is harmless to humans. Myxomatosis can result in lumps developing around the ears and face. These lumps are named myxomas and the disease virus was named after this lesion. […]
Diagnosis of Feline Respiratory Mycoplasma Infection

Bioguard Corporation In cats, ’mucosal’ mycoplasma infections typically cause ocular and respiratory disease, and less frequently neurological or joint disease. These Mycoplasma species are distinct to the haemotropic mycoplasmas that target red blood cells, causing hemolytic anemia in cats. Mycoplasma felis is typically associated with Upper Respiratory Tract Disease (URTD) in cats. Transmission M. felis […]
Tritrichomonas Infection in Cat
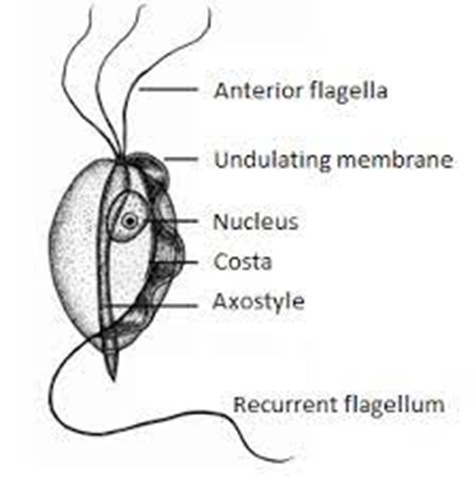
Bioguard Corporation Tritrichomonas foetus is a significant cause of large bowel diarrhea and persistent colitis in cats. These pear-shaped organisms have three anterior flagella and one posterior flagellum. They have a distinctive undulating membrane, which gives them a similar appearance to Giardia. However, they do not form cysts and are transmitted directly from one host […]
Respiratory Tract Disease Complex in Cats

Sushant Sadotra, PhD/Diagnostic specialist Feline respiratory disease (FRD) syndrome or feline upper respiratory tract disease complex is a common infection in cats caused mainly by Feline Herpesvirus (FHV-1), Feline Calicivirus (FCV), Chlamydophila felis, Mycoplasma spp., and Bordetella bronchiseptica. About 90% of all upper respiratory infections are caused by FHV-1 and FCV. Common Symptoms: · Sneezing · Nasal […]
Feline Herpesvirus Infection- Diagnosis

Trinh Mai Nguyen Tang Feline herpesvirus-1 (FHV-1) is a feline respiratory infection virus also known as feline viral rhinotracheitis (FVR) [1]. The Herpes virus was first isolated by scientists Crandell and Maurer in 1958 in cats with respiratory infections [2]. This virus has a prominent genome with large double stranded DNA, belonging to the […]
Canine Lyme Disease
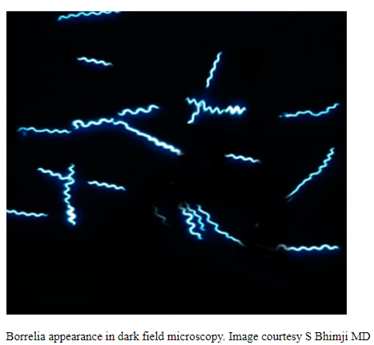
Oliver Organista, LA Lyme disease is a disease caused by the bacterium Borellia burdorgferi; a worm like, spiral-shape bacterium of spirochete class in the genus Borellia. The bacterium B. burgdorferi is transmitted through a bite of infected blacklegged tick or deer tick (Ixodes scapularis) to dogs and humans[1]. Different life-stage of I. scapularis ticks emerge at different […]
Psittacine Beak and Feather Disease

Long Pham Introduction Psittacine beak and feather disease (PBFD) is an infectious viral disease that infects psittacine birds. This disease affects Old World (Australian and African) psittacine birds and New World (Americas) psittacine birds (Greenacre, 2005). The peracute and acute form of this disease can cause sudden death, while the chronic form of this disease […]
Introduction to Feline Hypertrophic Cardiomyopathy
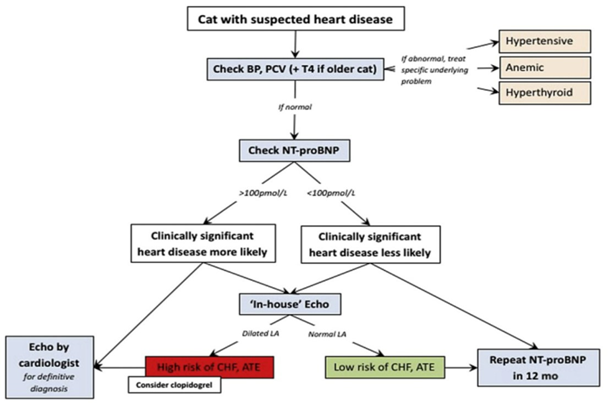
Maigan Espinili Maruquin It is important to be aware that some of the diseases your pets may have are actually inherited. In cats, there are myocardial diseases that can be breed- related. The most common myocardial disease in cats is Hypertrophic cardiomyopathy (HCM), wherein abnormal thickening of the walls of the left ventricle (LV) […]



