Factor VII Deficiency in Dogs

Bioguard Corporation Canine Factor VII (FVII) deficiency is an autosomal recessive genetic disorder that leads to a mild to moderate blood clotting problem in affected dogs. Puppies with the condition may exhibit symptoms such as nosebleeds (epistaxis) and gum bleeding, while adult dogs are more prone to bruising and skin issues like dermatitis. Though bleeding episodes tend to decrease in severity as the dog matures, the disorder still causes ongoing issues throughout the animal’s life. FVII deficiency was first identified in the Migluo breed. Most dogs are diagnosed when they visit a veterinarian due to accidental injury, spontaneous bleeding, genetic screening, or blood tests. Pathogenesis Hemostasis is achieved through a series of events known as the coagulation cascade, which involves two interconnected pathways: the intrinsic and extrinsic pathways. The intrinsic pathway is triggered by spontaneous internal damage to the blood vessel lining, while the extrinsic pathway is activated in response to external trauma. Factor VII (FVII) is a vitamin K-dependent glycoprotein produced in the liver and released into the bloodstream as a single-chain zymogen. Once activated, FVII plays a crucial role in initiating coagulation. Following vascular injury, FVII, along with tissue factor (TF) and calcium, activates factors IX and X, leading to thrombin production. A deficiency in FVII impairs blood clotting, resulting in excessive bleeding during injuries or surgeries. Diagnosis The Buccal Mucosal Bleeding Time (BMBT) is the most commonly used test for measuring bleeding time in small animals. To perform the BMBT, the upper lip is folded back and secured with a gauze strip around the maxilla or both the maxilla and mandible. A small incision is made in the mucosa above the premolars, avoiding areas with visibly engorged vessels. Blood from the incision is gently blotted using filter paper placed near the incision without touching it. A stopwatch starts when the incision is made and stops when no blood crescent forms on the filter paper. To reduce variability, the same person should perform the BMBT whenever possible. Activated Partial Thromboplastin Time (APTT) measures the overall speed of blood clot formation through the intrinsic and common coagulation pathways. It assesses the activity of factors XII, XI, IX, VIII, X, V, II, I, as well as prekallikrein (PK) and high molecular weight kininogen (HK). Prothrombin Time (PT) is used to evaluate the extrinsic and common pathways of coagulation by measuring factors VII, X, V, II, and I. Factor VII deficiency is suspected when a dog presents with a prolonged PT and normal BMBT and APTT. Genetic testing can also identify affected dogs or carriers, especially in certain breeds. Breeds at Risk Canine Factor VII deficiency has been documented in several breeds, including the Beagle, Airedale, Alaskan Klee Kai, American Foxhound, Finnish Hound, German Wirehaired Pointer, Giant Schnauzer, Irish Water Spaniel, Japanese Spitz, Miniature Schnauzer, Papillon/Phalene, Sealyham Terrier, Scottish Deerhound, and Welsh Springer Spaniel. Management There is currently no cure for Factor VII deficiency. However, clinical symptoms can be managed through transfusions with fresh plasma or blood, or by administering recombinant activated human FVII. These treatments provide only temporary relief. Fortunately, dogs with mild to moderate FVII deficiency typically lead normal lives. References Callan MB, Aljamali MN, Margaritis P, Griot-Wenk ME, Pollak ES, Werner P, Giger U, High KA. A novel missense mutation responsible for factor VII deficiency in research Beagle colonies. J Thromb Haemost. 2006 Dec; 4(12):2616-22. Carlstrom LP, Jens JK, Dobyns ME, Passage M, Dickson PI, Ellinwood NM. Inadvertent propagation of factor VII deficiency in a canine mucopolysaccharidosis type I research breeding colony. Comp Med. 2009 Aug;59(4):378-82. Donner J, Kaukonen M, Anderson H, Moller F, Kyostila K, Sankari S, Hytonen M, Giger U, Lohi H. Genetic Panel Screening of Nearly 100 Mutations Reveals New Insights into the Breed Distribution of Risk Variants for Canine Hereditary Disorders. PLoS One. 2016 Aug 15;11(8):e0161005. Kaae JA, Callan MB, Brooks MB. Hereditary factor VII deficiency in the Alaskan Klee Kai dog. J Vet Intern Med. 2007 Sep-Oct;21(5):976-81.
Pyruvate Kinase Deficiency in Dogs

Bioguard Corporation Pyruvate kinase deficiency (PKD) is a hereditary genetic disorder that impairs the ability of red blood cells to metabolize properly. This defect leads to the destruction of red blood cells, resulting in severe hemolytic anemia. Affected animals can die from complications such as severe anemia and liver failure. The condition typically manifests between 4 months and 4 years of age, with common clinical signs including weakness, increased heart rate, and heart murmurs. Pathogenesis In mammals, mature red blood cells lack mitochondria, which means they cannot produce energy through oxidative phosphorylation. Instead, they rely on glycolysis to generate ATP, which is essential for maintaining cell shape and active transport across cell membranes. Pyruvate kinase (PK) plays a key role in the final step of glycolysis, where it catalyzes the conversion of phosphoenolpyruvate into pyruvate, producing ATP in the process (as shown in the diagram below). When PK is deficient, red blood cells cannot synthesize sufficient ATP, leading to impaired cell metabolism. This energy deficit causes premature red blood cell death and results in hemolytic anemia. PKD is an autosomal recessive disorder caused by mutations in the PK-LR gene, which affects the activity of pyruvate kinase. Clinical Symptoms Pyruvate kinase deficiency (PKD) typically presents between 4 months and 4 years of age, causing severe chronic hemolytic anemia. Affected dogs may display symptoms such as exercise intolerance, severe limb weakness, easy fatigue, lethargy, underweight, pale gums, weight loss, emaciation, stunted growth, poor posture, and an increased heart rate. Ultrasound exams may reveal an enlarged liver and spleen, with common findings like bone sclerosis and hemosiderosis/hemochromatosis. Treatment and Prevention Currently, there is no effective drug treatment or way to slow the progression of PKD. The only treatment option is bone marrow transplantation, which may allow dogs to live a normal lifespan. However, this treatment is costly and carries a risk of death. Without treatment, affected dogs typically succumb to severe hemolytic anemia and liver failure. Genetic testing can identify PKD defects early in dogs and cats, enabling timely intervention and care. Affected Breeds Research indicates that certain dog breeds are more predisposed to inheriting PKD, including: Basenjis, Labrador Retrievers, Pugs, West Highland White Terriers, Cairn Terriers, Dachshunds, Terriers, Miniature Poodles, Chihuahuas, and American Huskies. Among cat breeds, Somali and Abyssinian cats are commonly affected, along with Egyptian Maus, LaPerms, American Shorthairs, Bengals, Maine Coons, Norwegian Forest Cats, Siberians, and Singapuras. Genetic Detection Genetic testing can determine whether dogs and cats carry PKD defects. Carriers may pass the gene on to offspring, so they should not be bred. If an animal tests positive for PKD, early monitoring and care are essential to manage the disease. References Chapman, B.L., & Giger, U. (1990). Inherited erythrocyte pyruvate kinase deficiency in the West Highland White Terrier. Journal of Small Animal Practice, 31, 610-616. Schaer, M., Harvey, J.W., Calderwood-Mays, M., & Giger, U. (1992). Pyruvate kinase deficiency causing hemolytic anemia with secondary hemochromatosis in a Cairn Terrier. Journal of the American Animal Hospital Association, 28(3), 233-239. Gultekin, G.I., Raj, K., Foureman, P., Lehman, S., Manhart, K., Abdulmalik, O., & Giger, U. (2012). Erythrocytic pyruvate kinase mutations causing hemolytic anemia, osteosclerosis, and secondary hemochromatosis in dogs. Journal of Veterinary Internal Medicine, 26(4), 935-944.
Juvenile Hereditary Cataracts in Dogs
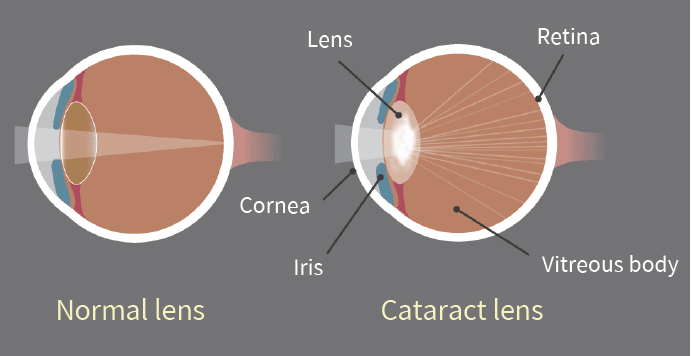
Bioguard Corporation Canine juvenile cataract (JHC) is a hereditary form of cataract characterized by cloudiness and degeneration of the lens in the eye. This condition prevents light and images from passing through the lens to the retina, impairing the dog’s vision and eventually leading to blindness. Affected dogs may exhibit symptoms such as bumping into furniture, losing direction, and moving slowly. JHC can also lead to intraocular complications, including uveitis, glaucoma, and lens luxation. Dogs are more prone to cataracts than any other species, and while cataracts can develop at any age, juvenile cataracts typically occur in dogs under 7 years old. Genetic mutaion related to JHC JHC is caused by a mutation in the heat shock transcription factor 4 (HSF4) gene, leading to an autosomal recessive inheritance pattern. Assuming that “A” represents a normal allele and “a” a mutated allele, an individual will exhibit symptoms of the disease only if both alleles are mutated (aa). Diagnosis A comprehensive ophthalmological examination is essential to assess the severity of cataracts and identify any associated complications. This examination typically includes tests for vision, pupillary light reflex, cataract severity and grading, and checks for any concurrent eye diseases or complications. Additional tests may include intraocular pressure measurement, tear production evaluation, corneal health assessment, fundus reflex and retinal examination, and ocular ultrasound. Genetic testing can further confirm the presence of genetic defects and is valuable as a pre-breeding health check to prevent the transmission of hereditary cataracts to offspring. Affected Breeds Breeds that are particularly prone to hereditary cataracts include Poodles, Cocker Spaniels, Boston Terriers, Siberian Huskies, Karelian Bears, Wire-haired Fox Terriers, Old English Sheepdogs, Golden Retrievers, and Labradors. It is recommended to conduct genetic testing before breeding these dogs to reduce the risk of producing affected offspring. Stages Cataracts can be classified into four stages based on the degree of lens opacity: Initial Stage: A distinct opaque white spot appears in the center of the pupil, but vision remains unaffected. Immature Stage: The lens begins to thicken both in the front and back, showing partial cloudiness. Vision becomes blurry, especially in low-light conditions, although some vision is still retained. Mature Stage: The entire lens becomes fully opaque and thickened, resulting in complete vision loss. Hypermature Stage: The clouded lens begins to shrink and clear up, making this stage prone to additional complications such as uveitis, glaucoma, and severe intraocular inflammation. This stage may also involve lens dislocation or fibrosis of the posterior capsule. Impacts and Complications The most noticeable early sign of cataracts is a change in eye color, where the lens begins to appear cloudy and white. It’s important to distinguish this from nuclear sclerosis, a common condition in older dogs. As the lens becomes cloudier, a dog’s vision deteriorates, leading to unintentional collisions with objects and increased sensitivity to the environment, often giving the dog a distant or blank stare. Cataracts typically worsen over time, resulting in a range of eye-related complications. These can include increased sensitivity, lens-induced uveitis (LIU), glaucoma, and the rupture of zonular fibers around the lens, which may cause lens dislocation or subluxation, as well as opacification of the posterior capsule. As cataracts progress to the hypermature stage, additional complications such as intraocular bleeding, retinal detachment, and further instances of glaucoma may arise. Early diagnosis and treatment are essential for managing these complications effectively and improving the overall prognosis. Diagnosis A comprehensive ophthalmological examination is essential to assess the severity of cataracts and identify any associated complications. This examination typically includes tests for vision, pupillary light reflex, cataract severity and grading, and checks for any concurrent eye diseases or complications. Additional tests may include intraocular pressure measurement, tear production evaluation, corneal health assessment, fundus reflex and retinal examination, and ocular ultrasound. Genetic testing can further confirm the presence of genetic defects and is valuable as a pre-breeding health check to prevent the transmission of hereditary cataracts to offspring. References Mellersh CS, McLaughlin B, Ahonen S, Pettitt L, Lohi H, Barnett KC. Mutation in HSF4 is associated with hereditary cataract in the Australian Shepherd. Vet Ophthalmol. 2009 Nov-Dec; 12(6):372-8. Mellersh CS, Pettitt L, Forman OP, Vaudin M, Barnett KC. Identification of mutations in HSF4 in dogs of three different breeds with hereditary cataracts. Vet Ophthalmol. 2006 Sep-Oct; 9(5):369-78.
Dog Frostbite: Canine Degenerative Spinal Neuropathy

Bioguard Corporation Degenerative Myelopathy, DM is a progressive chronic degenerative disease of the central nervous system, which is a recessive genetic disease that mainly occurs in the spinal nerve, similar to human amyotrophic lateral sclerosis. It is known as lou gehrig’s disease. The age of onset of dogs is between 9 and 11 years old, and the common diseased breeds will be explained later. Pathogenesis Current research points out that the cause of DM is highly correlated with the mutation of superoxide dismutase-1 (SOD 1) gene. SOD mainly plays the role of scavenging free radicals in the body. It is an important antioxidant enzyme system in the body. It can convert the more active superoxide (Superoxide, O2–) into the less active hydrogen peroxide ( H2O2) reduces excessive peroxide production in the body; however, when the SOD 1 in the dog’s body is genetically defective, free radicals cannot be eliminated. The accumulation of excessive free radicals will cause the death of motor neuron cells and produce a lot of degeneration disease. Clinical symptoms The progression of the disease can be divided into four stages based on its severity: First Stage: From complete proprioceptive dysfunction to upper motor neuron spastic paresis. Second Stage: From difficulty in standing and walking to complete paralysis of the hind limbs. Third Stage: From neuronal paralysis of the lower body to paraplegia affecting the forelimbs. Fourth Stage: From neuronal paralysis of the lower limbs to loss of brainstem function. Based on previous cases, the transition from stage 1 to stage 4 typically occurs within 6 to 9 months. The cause of the disease remains unknown, and there is currently no medication available for treatment. However, physical therapy and rehabilitation can help slow the progression of spinal cord degeneration and reduce limb atrophy. Early detection In the early stages, monitor your dog for any clinical symptoms and schedule an outpatient examination at a veterinary hospital. To diagnose degenerative myelopathy (DM), further testing may include assessing the dog’s breed, conducting neurological examinations, analyzing cerebrospinal fluid, performing X-rays, MRI scans, CT scans, and genetic testing. Early detection and treatment are crucial. Research on DM has identified degenerative spinal neuropathy in 115 dog breeds, with 48 breeds showing a high risk of SOD1 genetic defects. Note According to statistics from the American Orthopaedic Association, Corgis have the highest rate of abnormalities among all breeds. Since genetic testing began in 2008, a total of 4,428 Corgis have been tested, with the following results: 13.1% were normal, 33.6% were carriers, and 53.3% showed mutations.
Pancreatitis in Dogs

Dr. Sushant Sadotra Canine Pancreatitis is one of the most common endocrine diseases occurring in dogs. However, it is more prevalent in dog breeds such as Miniature Schnauzers, Yorkshire Terriers, Cocker Spaniels, Dachshunds, Poodles, and sled dogs. Pancreatitis, a severe inflammatory condition of the pancreas, can be short-term or long-term, based on the level of pancreatic tissue damage. It can be of acute or chronic type. It can be related to various clinical or subclinical signs and potentially life-threatening. Causes: Pathogenesis In the initial stages of Pancreatitis, the pancreatic juice is secreted in lesser amounts. Inside the pancreas, a series of steps lead to the activation of pancreatic enzymes. Co-localization of zymogen granules and lysosomes activates trypsinogen to trypsin, which further activates other zymogens. Premature activation of these digestive enzymes causes local damage such as edema, bleeding, inflammation, and necrosis of the pancreas. The inflammation process invites WBCs to the site and increases cytokine production. Altogether, this will cause further damage to pancreases and other distant complications such as generalized inflammation, disseminated intravascular coagulation, disseminated lipodystrophy, hypotension, renal failure, pulmonary failure, myocarditis, etc. Clinical Findings: Some of the most common symptoms in dogs are: The milder form of Pancreatitis can be related to subclinical or may have minor or nonspecific signs of anorexia, lethargy, or diarrhea. Diagnosis: Among all the methods discussed, histopathology is the gold standard for the diagnosis of canine Pancreatitis. However, a combination of mentioned techniques can be implemented in clinical practice for the most reliable and accurate diagnosis. Treatment: Careful monitoring and supportive veterinary care should be given in acute cases to prevent systemic complications. If a dog with chronic pancreatitis has no sign of improvement, additional trial therapy with an immunosuppressive agent such as prednisone, prednisolone, or cyclosporine may be prescribed for the treatment. Treatment for chronic pancreatitis is challenging because of systemic complications such as hypothermia, acidosis, hypocalcemia, and single- or multiple-organ failure. Reference: Watson, P. (2015), Pancreatitis in dogs and cats: definitions and pathophysiology. J Small Anim Pract, 56: 3-12. Whitley EM. Comparative Pancreatic Pathology. Pathobiology of Human Disease. 2014:1101–23. doi: 10.1016/B978-0-12-386456-7.03415-8. Epub 2014 Aug 21. PMCID: PMC7149520. Xenoulis, P.G. (2015), diagnosis of pancreatitis in dogs and cats. J Small Anim Pract, 56: 13-26.
Canine Chronic Hepatitis
Chavezlloyd Alexandria Baldago Chronic hepatitis is a condition in dogs that may occur due to various disease processes. It indicates a previous occurrence of inflammation and possibly cell death in the liver. The inflammation is caused by the infiltration of different types of white blood cells that are involved in the immune system. Necrosis, which refers to the death of a large number of liver cells, may also occur. The invasion of white blood cells and cell death in a dog’s liver can be due to previous damage caused by infectious agents like viruses or bacteria or as a result of toxic damage. Toxic damage may occur due to poisons ingested by the dog or abnormal accumulation of substances required by the body, such as copper. Though some breeds like Bedlington Terriers, Labrador Retrievers, Doberman Pinschers, Dalmatians, West Highland White Terriers, Welsh Corgis, Keeshonds, and others are notably affected, this toxicity can affect any breed of dog. Copper accumulation in hepatocytes can cause oxidative damage, and its presence can escalate hepatocyte damage caused by other factors. Until dietary recommendations are modified and implemented, this problem will continue to affect canines. Inflammation and cell death can also occur due to a primary attack by the immune system against liver cells, known as an “autoimmune” disease. Cancer in the liver is not called chronic hepatitis, even if it causes similar damage. Chronic vs Acute The term “chronic” refers to a condition that has been causing damage for a prolonged period, typically lasting several weeks or more. On the other hand, “acute” hepatitis is usually characterized by a shorter duration of just a few days. While some cases of acute hepatitis can be treated successfully, many types of chronic hepatitis are not curable. However, with appropriate treatment and close monitoring, a significant number of patients with chronic hepatitis can maintain a good quality of life with minimal clinical symptoms for an extended period. Chronic hepatitis can affect any breed of dog regardless of gender or age, although it is more common in middle-aged or older dogs. Certain breeds may be more prone to specific types of hepatitis. For instance, some breeds may develop chronic hepatitis due to the accumulation of copper in the liver cells. The excessive concentration of copper harms the liver cells and, if left untreated, typically leads to severe chronic hepatitis. Symptoms The liver has multiple functions, therefore the clinical signs associated with liver disease can vary significantly. Symptoms of hepatitis in dogs can include: Lack of appetite Lethargy Vomiting Increased urination Excessive thirst and urination Swollen belly filled with fluid (ascites) Yellowish gums (jaundiced) and moist tissues Seizures, mental dullness Diagnosis Apart from obtaining a detailed history of the affected dog’s health prior to the appearance of symptoms, the next step would be to conduct a comprehensive physical examination of the dog, which includes carrying out a blood chemical profile, a complete blood count, an electrolyte panel, and a urinalysis. The results of the bloodwork will enable your veterinarian to identify any signs of impaired kidney function. To further evaluate the disease, complete abdominal ultrasonography is essential. This diagnostic tool screens for concurrent diseases and helps acquire bile. It’s important to note that the liver can appear normal upon examination, and changes seen in chronic hepatitis can include uniform increases in liver echogenicity, decreased distinction of portal vein margins, and normal to small liver size. Abnormalities in the liver parenchyma, biliary tree, portal vein, and peritoneum should be assessed, along with acquired shunting and the presence of free peritoneal fluid. In order to accurately diagnose chronic hepatitis, a liver biopsy is necessary. There are 3 ways to perform a liver biopsy, surgically, via laparoscopy, or through the skin using a special needle under ultrasound guidance. However, surgical or laparoscopic biopsies are more informative compared to ultrasound-guided biopsies. The information obtained from the biopsy is required to determine the type and severity of liver disease, as well as to assess your dog’s prognosis. Treatment The treatment for chronic hepatitis in dogs is a complex process that depends on the severity and type of liver disease, as well as the clinical signs exhibited by the dog. In severe cases, hospitalization, intravenous fluid therapy, and supportive care may be required. The most common medications prescribed for treating the disease are immunosuppressive or anti-inflammatory. In some cases, dietary changes may be necessary. Certain medications may also be used in specific situations, such as dogs whose illness is linked to copper accumulation, abdominal fluid build-up, or neurological symptoms. Life Expectancy Although this condition cannot be cured with the available treatments, the good news is that the dog can still live a good quality of life for months and even years with continued therapy. Regular blood work is necessary to ensure the dog is responding well to the treatment. This helps to adjust the medication and keep the dog relatively free of clinical signs. References: https://bluepearlvet.com/medical-articles-for-pet-owners/chronic-hepatitis-in-dogs/ https://todaysveterinarypractice.com/hepatology/canine-chronic-hepatitis-diagnosis-treatment/ https://www.msdvetmanual.com/digestive-system/hepatic-diseases-of-small-animals/canine-chronic-hepatitis#:~:text=Canine%20chronic%20hepatitis%20is%20a,be%20predisposed%20to%20chronic%20hepatitis.



